ChemAlive Activities and News
March, 2021
ChemAlive gives a lecture on digital chemistry and sustainability at BIO-NRW
PResenting all our contract work with direct impact on sustainability in the chemical industry. Scan to 1h 31 m to see Peter's talk.
February, 2021
ChemAlive Pitches with 5HT Digital Hub Chemistry
Presenting our latest market positioning for client engagement and pilots.
July, 2019
Collaborative Paper With Aramco Services Published in Polymers
Abstract
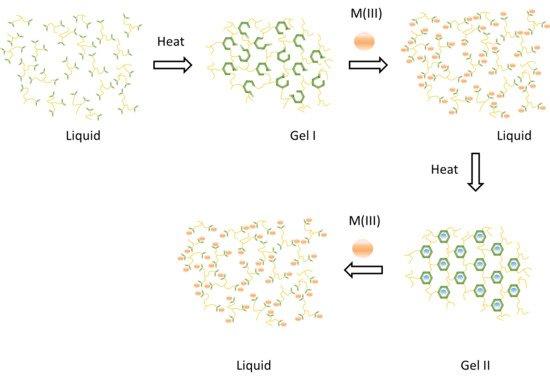
In this article, we review a dynamic covalent gel system developed as a high temperature well construction fluid. The key gel/fluid phase changes and related materials properties are addressable via the constitutional and coordination dynamics of the equilibrium and non-equilibrium molecular species comprising the material. The interplay between these species and external stimuli leads to material adaptability. Specifically, the introduction of metal ions into a non-equilibrium hemiaminal gel reverts this phase into a non-equilibrium liquid. When heated, this liquid transforms itself catalytically into the thermodynamically favoured closed-ring polyhexahydrotriazine (PHT) gel product. The temperature stability of different PHT gel formulations is evaluated as a function of the inclusion of various salts. It is possible to revert this thermodynamic PHT gel back into a liquid. This pH dependent transformation depends on the R groups linking the hexahydrotriazines (HTs) to one another. While polyethylene glycol (PEG) based PHT gels revert to liquids with water and mild protonation conditions, in comparison, polypropylene glycol (PPG) based gels require stronger acid conditions with heat, or a different more nucleophilically driven ring-opening mechanism by, for example, phosphines. The covalent dynamic chemistry in this chemical system gives way to many possible applications in addition to the high temperature solution-gelation (sol-gels) for which it has been primarily designed.
June, 2019
BiopharmaTrend Blog Post
ChemAlive discussed as one of the new start-ups in quantum chemical space.
May, 2019
Collaborative Paper With University of Sophia
Abstract
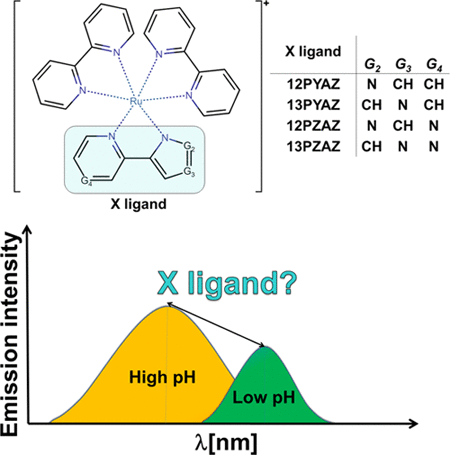
Three new [Ru(bpy)2X]+ complex ions, where bpy represents bipyridyl ligand and X denotes pyridyl diazolate or pyrazinyl diazolate coordination site, have been computationally designed and synthesized as pH-sensitive molecules. The choice of pyridyl and pyrazinyl moieties allows for the nitrogen content to vary, whereas the influence of the protonation site is quantified by using 1,2-diazolate and 1,3-diazolate derivatives. The absorption and emission properties of the deprotonated and protonated complex ions were characterized by UV–vis and photoluminescence spectroscopy as well as by time-dependent density functional theory. Protonation causes (1) a strong blue shift in the lowest energy 3MLCT → S0 emission wavelengths, (2) a substantial increase in the emission intensity, and (3) a change in the character of the corresponding 3MLCT emitting states. The blue shift in the emission wavelength becomes less pronounced when the nitrogen content in the X-ligand increases and when going from 1,2- to 1,3-diazolate derivatives. The contrast in the emission intensity of the protonated/deprotonated forms is the highest for the complex ion, containing a 2-pyridyl derivative of the 1,2-diazolate. The complex ions are suggested as potential pH-responsive materials based on change in the color and intensity of the emitted radiation. The broad impact of the research demonstrates that the modification of the nitrogen content and position within the protonable ligands is an effective approach for modulation of the pH-optosensing properties of Ru–polypyridyl complexes.
March, 2019
Our collaboration with Aramco Services on dynamic metallogels and their reversion with phosphine is out on MRS Communications
Title
Dynamic covalent hexahydrotriazine breakdown through nucleophilic attack by phosphine
Abstract
In the current manuscript we discuss the response of dynamic metallogels that display reversion to the liquid state when exposed to phosphines. The metallogels are formed through the condensation of formaldehyde and poly(alkyloxide)amines in polar aprotic solvents. The gel formation can be catalyzed with trivalent metals (Al(III and Fe(III)) with concomitant enhanced dynamism (gelation/degelation). When various phosphines are introduced, the metallogel is irreversibly liquefied. This process adds a new vector for controlling the bulk properties of this class of materials. Here, we explore the mechanism in detail for the reaction of tris(carboxyethyl)phosphine with N,N,N-triethoxylethyl-1,3,5-hexahydro-1,3,5-triazine (HEHT, 1) a stable derivative of the active hexahydrotriazine (HT) core in dimethylformamide in the presence or absence of Al(III). Additionally, density functional theory is used on the model N,N,N-trimethyl system (MHT, 2) to estimate reaction parameters and predict nuclear magnetic resonance spectra.
December, 2018
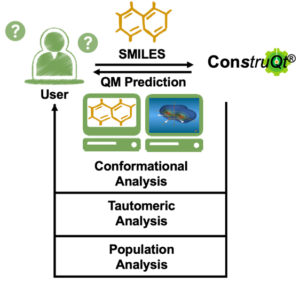
BiopharmaTrend Blog Post
ConstruQt big data analysis covering conformational and tautomeric analysis
June, 2018
ChemAlive Innosuisse Grant Interview
ChemAlive - MARVEL collaboration wins grant to boost machine learning approaches to real-time chemical and reaction modelling
June, 2018
ChemAlive gives webinar to JnJ
Title: "Accelerating Access to More Physical Quantum Chemical Data for Ligand-Based Virtual Library Screening through Machine Learning"
Abstract: "The ChemAlive engine and API brings machine learning and quantum chemistry to the chemical and pharma industry, allowing the modeling of accurate quantum chemical metrics at scale for the design, prediction and understanding of chemical properties. By leveraging machine learning techniques we are able to deliver quantum mechanical accuracy at classical mechanics speeds including improved atomic charges and conformational structure. With quantum we can quickly assess tautomeric and diastereomeric space as well as solvation effects using a strict molecular energy regime rather than a rules-based regime. ChemAlive is looking for large-scale drug discovery partners to deploy its technology."
June, 2018
ChemAlive gives Plenary Lecture at Evotec (Toulouse)
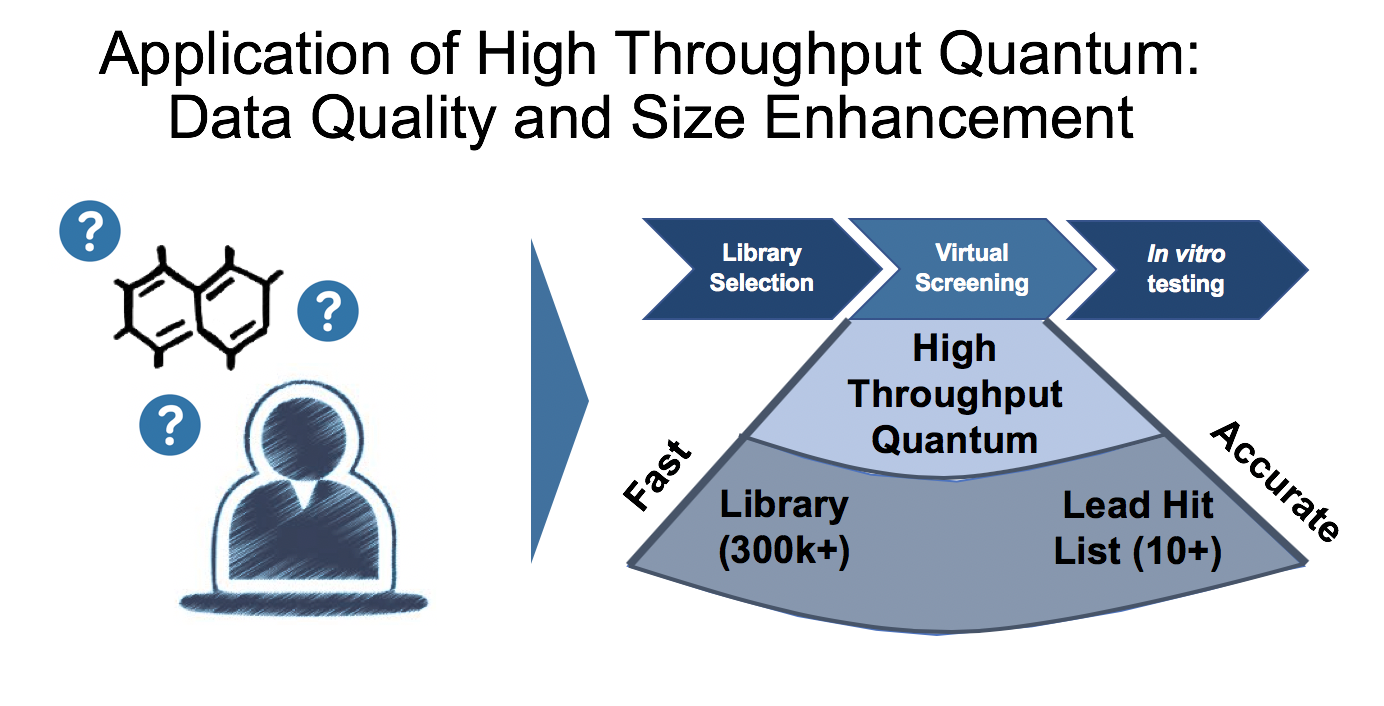
Title: "Reaction Prediction (Screening) Coupled to Virtual Library Data through High Throughput Quantum and Machine Learning"
Abstract: "Reaction prediction has traditionally been based on encoding of chemical derived rules from experience, models and, more recently, literature data. Next generation approaches coming out of academic work have begun to use more physics-based and quantitative measures, such as atomic charges, but still rely on the known to predict the unknown. ChemAlive is working towards a fully quantum and physical data driven reaction prediction regime that unlocks the large unknown chemical space where high value intellectual property is likely to be found. To access qualitatively reliable quantum chemical data in drug discovery, quantum chemistry itself must scale. ChemAlive is developing a high throughput solution to address reaction prediction (screening) at the lead (cloud computations) and library (machine learning) scale."
June, 2018
PM @Theresa_May hosted a reception at Downing Street as part of London Tech Week, celebrating UK's position as a world-leading destination for tech investment #LTW #Techat10 pic.twitter.com/WNwCaffMFQ
— UK Prime Minister (@10DowningStreet) June 15, 2018
ChemAlive invited to 10 Downing Street as a leading tech company to an address by UK Prime Minister on Tech in Britain
Thanks so much to Founders Factory for arranging this event
March, 2018

ChemAlive's CEO Gives Lecture at UCLA
Peter returns as an alumnus to give a lecture on ChemAlive activities to the UCLA chemistry department with focus on turning chemical technology into deeptech business
February, 2018
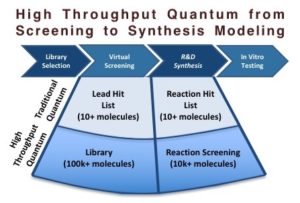
ChemAlive Releases White Paper
High Throughput Quantum Chemistry for Drug Discovery - Towards Reaction Screening - was published as a white paper on BioPharmaTrend.com a leading channel for drug discovery, pharma and biotech viewpoints.
January, 2018
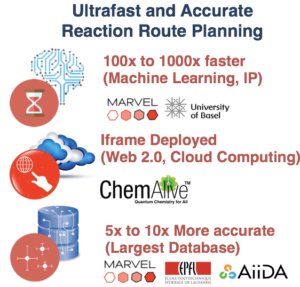
ChemAlive Receives CTI (Innosuisse) Grant
ChemAlive have been awarded a InnoSuisse Grant jointly with academic partners EPFL and UniBasel. Starting in Q2, 2018 the project will run for 1.5 years through 2019.
December, 2017
Slush: Climate Impact Battle
ChemAlive made top 10 of the Climate-KIC start-ups Europe wide to present at the SLUSH investor conference in Helsinki.

November, 2017
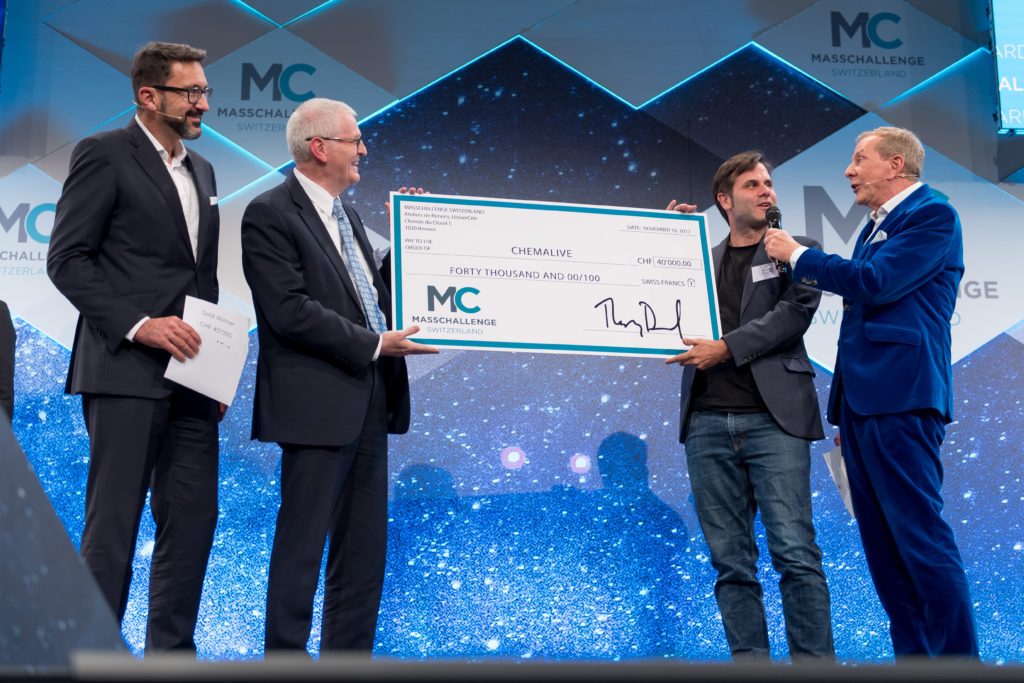
MassChallenge Gold Winners
On Thursday 16th November, ChemAlive were announced as Gold Winners of the MassChallenge start-up competition!

October, 2017
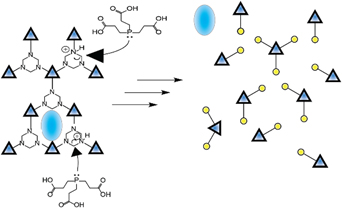
Publication: Journal of American Chemical Society
DOI: 10.1021/jacs.7b07053
Gels produced with dynamic polymers are broken down to liquids after the addition of metal salts. NMR studies and DFT calculations reveal that the energetic landscape render stable nonequilibrium products. These species remain stable in the liquid phase at room temperature but convert to gels upon heating. Controlled gel times dictated by the thermodynamics and kinetics of the system were achieved harnessing self-healing properties and triggered release functionality.
October, 2016

ChemAlive Enters Climate-KIC Accelerator
EIT Climate-KIC is a European knowledge and innovation community, working towards a prosperous, inclusive, climate-resilient society founded on a circular, zero-carbon economy